folgende Studie gefunden:
https://www.ncbi.nlm.nih.gov/pubmed/23979207
Can combination metronomic therapy overcome chemoresistance in cholangiocarcinoma? A literature review
Prognose für CholangioCa ist sehr schlecht. Im Fallbericht wird eine 55-year-old woman präsentiert mit multifocal iCCa. Sie lehnte standard-Chemo ab und bekam daher metronomic therapy mit combination of celecoxib, etoposide, and cyclophosphamide for a total of 30 months.
Derzeit nach 57 Monaten (und 27 Monate nach dem alle Behandlungen abgesetzt wurden) befindet sie sich immer noch in einer kompletten radiologischen Remission
Hier der wichtigste Aussschnitt aus dem Fallbericht
She was started on oral chemotherapy with celecoxib 200 mg twice daily (BD) everyday along with etoposide 50 mg once daily (OD), and cyclophosphamide 50 mg OD for 21 days every 28 days.
- Vepesid-Weichkapseln OP1/10 ca 500,-
- –> besser magistrale Abfüllung auf 50mg Etoposid in der Apotheke anfertigen lassen, “Etoposid 50mg Kapseln, OP1 / 126 Kapseln”
- Celecoxib 200mg OP1/30 ca 15,-
- Endoxan 50mg Dragees OP1/50 19,-
She received six such cycles, and an ultrasound of the abdomen done in April 2009 revealed that there was a partial response in the bigger lesion which had decreased to 25 × 19 mm with a few calcified foci in that lesion. The smaller lesion in the left lobe had completely resolved.
Because of financial reasons, oral etoposide was stopped and she was continued on just oral celecoxib and cyclophosphamide for another two years (with occasional periods of either drug being omitted because of financial reasons). A repeat ultrasound done nearly one year from diagnosis in December 2009 showed no evidence of the lesions with only a few calcific foci. The patient has been off treatment and on regular follow-up since April 2011 and the latest CT scan done on 19 January 2013 showed that the patient continues to be in complete remission
damit bekommen wir erstmals bei diesem gefürchteten Tumor der schlecht auf die normale Chemo anspricht eine vertretbare und leistbare Therapie in die Hand die uns so etwas wie “Heilung” verspricht.
Weil der Artikel (FullText) im Indischen Journal keine stabile URL hat, hab ich ihn bis auf Widerruf hier reinkopiert:
| » Introduction |  |
Cholangiocarcinoma (CCa) is a tumor that originates from the neoplastic transformation of the epithelial cells of the intrahepatic or extrahepatic bile ducts. This type of cancer is difficult to diagnose, extremely aggressive, and has very poor prognosis. [1] It is also relatively resistant to chemotherapy and radiotherapy, and the most effective therapy is complete surgical resection. [1] However, in most patients, diagnosis is very late; complete surgical resection is not possible and palliation is the main stay of treatment.
Recent data shows that the tumor microenvironment is a very important factor in the regulation of angiogenesis, invasion, and metastasis of the tumor, and may play a role in the pathogenesis and classification of CCa. [1] Thus metronomic therapies, which are known to be directed toward the tumor microenvironment [2] may play an important role in improving the outcome of patients with CCa.
Here, we report a case of multifocal intrahepatic CCa (iCCa) who achieved complete long-lasting remission on treatment with metronomic combination therapy of oral celecoxib, etoposide, and cyclophosphamide. Appropriate literature justifying a combination of biological treatment with chemotherapeutic agents to improve the outcome of patients with CCa has been reviewed. References for the review were identified through searches of Pubmed with the search terms ‘cholangiocarcinoma’, ‘therapy’, ‘metronomic’, and ‘COX II’ alone or in combination, for the last 10 years. Articles were also identified through searches of the files of the authors themselves. Only papers published in English were included. The final list was generated on the basis of originality and relevance to this review.
| » Case Report |  |
A 55-year-old female presented at the BKL Walawalkar Hospital, the rural outreach center of Tata Memorial Hospital, on 21 July 2008 with a history of significant pain in the right hyprochondriac region of a duration of four to five days. There was no history of fever or vomiting. The hemogram and liver and kidney function tests were within normal limits. A computed tomography (CT) scan of the abdomen showed 35 × 35 mm well-defined, rounded hypodense lesion showing heterogeneous enhancement in the segment VIII of the right lobe of the liver. Another subcentimeter-sized peripherally enhancing lesion was seen in the segment IV of the right lobe of the liver just next to the falciparum ligament [Figure 1]a and b. A CT-guided biopsy of the liver lesion confirmed the diagnosis of CCa. Thus, the patient was diagnosed to have multifocal iCCa. She did not have the known predisposing factors for developing iCCa, such as cirrhosis or infective viral hepatitis. Considering her socioeconomic status, the diagnosis, prognosis, and treatment with standard injectable gemcitabine plus cisplatin chemotherapy versus oral palliative chemotherapy was discussed with the patient and her relatives, and the patient opted for oral palliative chemotherapy.
 |
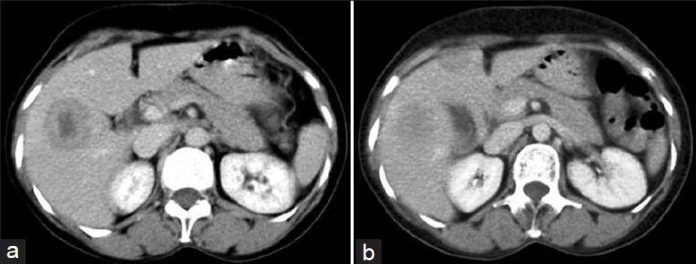
Figure 1: (a) Computed tomography scan dated 12 July 2008 shows two lesions of multicentric intrahepatic cholangiocarcinaoma. (b) Computed tomography scan dated 12 July 2008 shows two lesions of multicentric intrahepatic cholangiocarcinaoma |
She was started on oral chemotherapy with celecoxib 200 mg twice daily (BD) everyday along with etoposide 50 mg once daily (OD), and cyclophosphamide 50 mg OD for 21 days every 28 days. She received six such cycles, and an ultrasound of the abdomen done in April 2009 revealed that there was a partial response in the bigger lesion which had decreased to 25 × 19 mm with a few calcified foci in that lesion. The smaller lesion in the left lobe had completely resolved. Because of financial reasons, oral etoposide was stopped and she was continued on just oral celecoxib and cyclophosphamide for another two years (with occasional periods of either drug being omitted because of financial reasons). A repeat ultrasound done nearly one year from diagnosis in December 2009 showed no evidence of the lesions with only a few calcific foci. The patient has been off treatment and on regular follow-up since April 2011 and the latest CT scan done on 19 January 2013 showed that the patient continues to be in complete remission [Figure 2]a and b.
Overall, the oral metronomic chemotherapy was well tolerated except for one episode of grade II stomatitis and one episode of herpes zoster (T9 dermatome), both while on celecoxib/etoposide/cyclophosphamide combination. There was no hematological or biochemical toxicity noted.
 |
Figure 2: (a) Same cuts as in Figures 1a and b of computed tomography scan dated 19 January 2013 showing continuous complete remission. (b) Same cuts as in Figures 1a and b of computed tomography scan dated 19 January 2013 showing continuous complete remission |
| » Discussion |  |
CCa originates from the neoplastic transformation of cholangiocytes into intrahetpatic, perihilar, or distal extrahepatic tumors. [3] Treatment options for iCCa are limited with high rates of tumor recurrence and short survival times. [4] Where surgical resection can be offered, the median survival time is 36 months with a recurrence rate of 62.2% after a median of 26 months of follow-up evaluation. [5] Multifocal tumors have high rates of recurrence (>90%) and usually preclude curative resection. [5]
Systemic chemotherapy is the treatment of choice for inoperable (advanced and metastatic) CCa. The Advanced Biliary Cancer study (ABC-02) showed that as compared to single-agent gemcitabine, systemic chemotherapy with a combination of gemcitabine and cisplatin prolonged survival times of patients with inoperable CCa [response rate (RR) 15 vs. 26%; overall survival (OS) 8.1 vs. 11.7 months)] making it a treatment standard. [6] Though this treatment was proposed to our patient, because of socioeconomic and logistic reasons, she did not accept this treatment. A recent meta-analysis also supports adjuvant treatment for patients with lymph node-positive disease. [7] According to phase II and III trials, regimens combining 5-fluorouracil (5-FU) or gemcitabine with a platinum salt have provided an overall RR of 12 to 50% and a median OS of 5 to 16 months. [8],[9] Similar results are also available for gemcitabine plus oxaliplatin-containing regimen (OS of 10 months for solitary tumors, and 7 months for multifocal tumors). [8]
Though chemoradiation with gemcitabine is safe and can be applied in patients with R1 resection (with microscopic margin involvement) resection or unresectable CCa, the reported outcome maybe similar to patients with unresected tumor receiving combination chemotherapy with gemcitabine plus platinum. [10] Other potential therapeutic options for inoperable iCCa without extrahepatic metastasis include transarterial chemoembolization (TACE), radiofrequency ablation (RFA), and transarterial radioembolization (TARE). Patients who receive TACE or TARE have median survival times of 20.0 and 43.7 months, respectively, after diagnosis. [11],[12] For patients amenable to local therapy, this approach might be a palliative treatment option – especially for patients whose performance status (a major prognostic factor) precludes more aggressive approaches. [4] However, these options were again not available to our patient because of both socioeconomic reasons as well as their nonavailability locally. In some patients with advanced tumors, procedures like stenting and photodynamic therapy as well as new ablative techniques have also been used as palliative therapies. Although liver transplant might seem to be a good option for patients with iCCa, the median time of disease-free survival is only eight months and the five-year rate of tumor recurrence post transplantation is higher than 70%, which is unacceptably high. [13]
Various biological agents have been used as monotherapies for patients with CCa with varying results – sorafenib, erlotinib, lapatinib, sirolimus, and imatinib have disappointingly led to OS of 4.4, 7.5, 5.2, 7, and 4.9 months, respectively. Indeed, the outcomes were consistently inferior to those of standard combination chemotherapy with gemcitabine plus cisplatin. Of note, RRs were better when biological agents were used in combination – bevacizumab and erlotinib (RR: 12%; disease control rate (DCR): 69%; OS: 9.9 months) and even better when a biological agent was combined with chemotherapy. For instance, interferon alpha (IFNα) plus 5-FU or bevacizumab plus gemcitabine and oxaliplatin, respectively, yielded RR of 34 and 40% (DCR 69%), and OS of 12 months and 12.7 months. [14] The preliminary results of an ongoing study with gemcitabine and oxaliplatin with cetuximab showed a high RR of 63% and OS of 12.7 months. [15] A new drug, MEK 1/2 inhibitor, selumetinib showed an RR of 12% and DCR of 80%. [16] A study by Paule et al., in particular indicates that cetuximab may revert chemoresistance, as responses were seen when adding cetuximab to a chemotherapy regime (gemcitabine and oxaliplatin) on which the disease had progressed. [17]
It has been postulated that the tumor microenvironment plays a role in the growth, progression, and metastatic invasion in CCa. [1],[18],[19] The interaction between stromal and CCa cells via signaling mediators results in an environment that supports the growth of the tumor and suppresses innate immunity, thus conferring resistance to cytotoxic insults (endogenous and chemotherapeutic). [1],[20],[21] Targeting the tumor microenvironment along with the CCa cells directly may lead to more effective therapeutic strategies to treat this challenging cancer. Chronic inflammation and CCa seem to be intimately related. [22]CCa cells are known to overproduce many inflammatory cytokines leading to an increase in the number of tumor-associated macrophages (TAMs). These TAMs in turn cause infiltration of FOX P3 plus regulatory cells within the tumor. Also, TAMs may play a role in the progression of CCa, and regulatory T cells (T-regs) are known to contribute to the resistance to chemotherapy as well as radiotherapy. When our patient opted for palliative treatment only, a combination targeting the microenvironment including angiogenesis, inflammation, and tumor cells was considered in line with the intrinsic multitarget nature of metronomic chemotherapy. [23],[24] Keeping the palliative nature of treatment, only oral and low-cost drugs were considered which required minimum follow-up investigations. Thus, a combination of oral celecoxib, cyclophosphamide, and etoposide was given to our patient.
As we are aware, CCa is markedly resistant to chemotherapy, but the mechanism of drug resistance is not fully known. The expression of cyclooxygenase 2 (COX-2) is increased in human cancers including CCa. [25] Overexpression of COX-2 was shown to enhance growth of human CCa cells. [25] Data indicates that celecoxib, a COX-2 inhibitor preferentially induces apoptosis in CCa cells through COX-dependent mechanism and PGE-2 pathway [26] through a mechanism involving AKT inactivation, Bax translocation, and cytochrome-C release. [27],[28] In a study reported by Watkins et al., when celecoxib was added to a chemotherapy regimen of gemcitabine and irinotecan, a 40% RR was noted and an OS of 17 months was achieved. [29] Celecoxib was noted to induce a dose-dependent inhibition of cell growth, cell cycle arrest at the G1-S checkpoint, and induction of cyclin-dependent kinase inhibitors P21 (waf 1/cip1) and P 27 (Kip 1). [25] The expression of P 27 (Kip 1) was shown to enhance the apoptosis and growth inhibition of CCa cell lines (QBC-939) inducted by cyclophosphamide. [30] Thus, addition of celecoxib may remarkably increase the drug sensitivity of CCa cells to chemotherapy drugs like cyclophosphamide [30] and etoposide. [31]Our patient may have achieved long-term remission through this mechanism. The anticancer agent etoposide was added, keeping in mind the fact that oral etoposide has already been used in palliative regimens in various adult and pediatric solid tumors. [31] Etoposide is also antiangiogenic and is synergestic when given with celecoxib. [31] Also, as many CCa patients may have abnormal liver function tests (LFTs), etoposide would be a relatively safer drug to be given in such settings. Cyclophosphamide additionally depletes T-regs and is also antiangiogenic.
In summary, the nonsurgical treatment of CCa is rapidly evolving and a multidisciplinary approach is the cornerstone of planning optimal treatment. The combination of cisplatin and gemcitabine is considered as the standard of care for inoperable CCa. However, there is plenty of room for improvement. Though biological targeted treatment has minor effects when given as monotherapy, it is more effective when given in combination with chemotherapy. We need more knowledge about the specific effects of biological treatment and the optimal combination of biological and cytotoxic agents. [14] The current literature in this field, including this report, is characterized by mostly small studies and case reports, which are hypothesis generating at the best, but misleading at the worst. We plan to explore such therapies in patients at the Tata Memorial Hospital. However, considering the rarity of this disease, well-designed randomized international trials as well as marker-driven therapies seem mandatory for progress in treatment within a reasonable time in CCa. The present framework of developing more effective therapies is to first find the most efficient cytotoxic agent, second, the most efficient combination of cytotoxic agents, and third, to boost the effect by adding a biological compound. [14] This is exactly what we did in our patient effectively and it highlights the potential of metronomics, the science of combining metronomic chemotherapies and drug repositioning. [24]
| » References |  |
| 1. | Leyva-Illades D, McMillin M, Quinn M, DeMorrow S. Cholangiocarcinoma Pathogenesis: Role of the tumor microenvironment. Trans Gastrointest Cancer 2012;1:71-80.  |
| 2. | Pasquier E, Kavallaris M, André N. Metronomic chemotherapy: New rationale for new directions. Nature Rev Clin Oncol 2010;7:455-65.  |
| 3. | Alpini G, Prall R, La Russo N. The pathobiology of biliary epithelia. In: Arias I, Boyer J, Chisari F, editors. The liver; biology and pathobiology. Philadelphia: Lippincott Williams and Wilkins; 2001. p. 421-35.  |
| 4. | Razumilava N, Gores GJ. Classification, diagnosis and management of cholangio carcinoma. Clin Gastroenterol Hepatol 2013;11:13-21.  [PUBMED] |
| 5. | Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra, et al. Intrahepatic cholangiocarcinoma: Rising frequency, improved survival and determinants of outcome after resection. Ann Surg 2008;248:84-96.  |
| 6. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81.  [PUBMED] |
| 7. | Horgan AM, Amir E, Walter T, Knoxx JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta analysis. J Clin Oncol 2012;30:1934-40.  |
| 8. | Pracht M, Leu Roux G, Sulpice L, Mesbah H, Manfredi S, Audrain O, et al. Chemotherapy for inoperable advanced or metastatic cholangiocarcinoma: Retrospective analysis of 78 cases in a single center over four years. Chemotherapy 2012;58:134-41.  |
| 9. | Eckmann KR, Patel DK, Landgraf A, Slade JH, Lin E, Kaur H, et al. Chemotherapy outcomes for the treatment of unresectable intrahepatic and hilar cholangiocarcinoma: A retrospective analysis. Gastrointest Cancer Res 2011;4:155-60.  |
| 10. | Habermehl D, Lindel K, Rieken S, Haase K, Goeppert B, Buchler MW, et al. Chemoradiation in patients with unresectable extrahepatic and hilar cholangiocarcinoma or at high risk for disease recurrence after resection: Analysis of treatment efficacy and failure in patients receiving postoperative or primary chemoradiation. Strahlenther Onkol 2012;188:795-801.  |
| 11. | Kiefer MV, Albert M, McNally M, Robertson M, Sun W, Fraker D, et al. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol and polyvinyl alcohol: A 2-center study. Cancer 2011;117:1498-505.  |
| 12. | Hoffmann RT, Paprottka PM, Schon A, Bamberg F, Hauq A, Durr EM, et al. Transarterial hepatic ytrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: Factors associated with prolonged survival. Cardiovasc Intervent Radiol 2012;35:105-16.  |
| 13. | Sapisochin G, Fidelman N, Roberts JP, Yao FY. Mixed hepatocellular cholangiocarcinoma and intrahepatic cholangiocarcinoma in patients undergoing transplantation for hepatocellular carcinoma. Liver Transpl 2011;17:934-42.  [PUBMED] |
| 14. | Jensen L H and Jakobsen A. Combining biological agents and chemotherapy in the treatment of colangiocarcinoma. Expert Rev. Anticancer Ther 2011;11:589-600.  |
| 15. | Gruenberger B, Schueller J, Heubrandtner U, Wrba F, Tamandl D, Kaczirek K, et al. Cetuximab, gemcitabine and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: A phase II study. Lancet Oncol 2010;11:1142-8.  [PUBMED] |
| 16. | Bekaii-Saab T, Phelps M, Li X, Saji M, Goff L, Kauh JS, et al. A multi institutional Phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol 2011;2357-63.  |
| 17. | Paule B, Herelle MO, Rage E, Ducreux M, Adam M, Guettier C, et al. Cetuximab plus gemcitabine-oxaliplatin in patients with refractory advanced intrahepatic cholangiocarcinomas. Oncology 2007;72:105-10.  |
| 18. | Bhowmick NA, Neilson EG, Mosel HL. Stromal fibroblasts in cancer initiation and progression. Nature 2004;432:332-7.  |
| 19. | Tisty TD. Stromal cells can contribute oncogenic signals. Semin Cancer Biol 2001;11:97-104.  |
| 20. | deVisser KE, Jonkers J. Towards understanding the role of cancer-associated inflammation in chemoresistance. Curr Pharm Des 2009;156:1844-53.  |
| 21. | Shinohara ET, Maity A. Increasing sensitivity to radiotherapy and chemotherapy by using novel biological agents that alter the tumor microenvironment. Curr Mol Med 2009;9:1034-45.  [PUBMED] |
| 22. | Mantovani A, Sica A. Macrophages, innate immunity and cancer: Balance, tolerance and diversity. Curr Opin Immunol 2010;22:231-7.  [PUBMED] |
| 23. | Pasquier E, Kavallaris M, André N. Metronomic chemotherapy: New rationale for new directions. Nature Rev Clin Oncol 2010;7:455-65.  |
| 24. | André N, Banavali S, Snihur Y, Pasquier E. Time for Metronomics in Developing Countries? Lancet Oncol 2013;14:e239-48.  |
| 25. | Han C, Leng J, Demetris AJ, Wu T. Cyclooxygenase-2 promotes human cholangiocarcinoma growth: Evidence for cyclooxygenase-2 independent mechanism in celecoxib-mediated induction of P21waf1/cip1and p27kip1 and cell cycle arrest. Cancer Res 2004;64:1369-76.  [PUBMED] |
| 26. | Wu GS, Zou SQ, Llu ZR, Tang ZH, Wang JH. Celecoxib inhibits proliferation and induces apoptosis via prostaglandin E2 pathway in human cholangiocarcinoma cell lines. World J Gastroenterol 2003;9:1302-6.  |
| 27. | Zhang Z, Lai GH, Sirica AE. Celecoxib-induced apoptosis in rat cholangiocarcinoma cells mediated by AKT inactivation and Bax translocation. Hepatology 2004;39:1028-37.  [PUBMED] |
| 28. | Wu T, Leng J, Han C, Demetris AJ. The cyclooxygenase-2 inhibitor celecoxib blocks phosphorylation of Akt and induces apoptosis in human cholangiocarcinoma cells. Mol Cancer Ther 2004;3:299-307.  [PUBMED] |
| 29. | Watkins JF, Mayo MS, Smith HJ, Williamson SK. Gemcitabine, irinotecan and celecoxib in patients with biliary cancer. Anticancer Drugs 2009;20:294-300.  [PUBMED] |
| 30. | Luo J, Cao ZH, Lu MF, Zuo S, Dong JQ, Zou SQ. Effect of adenovirus-mediated mutant exogenous P27kip1 gene expression on the chemosensitivities of cholangiocarcinoma cell line. Zhonghua Wai Ke Za Zhi 2006;44:1349-52.  |
| 31. | Panigrahy D, Kaipainen A, Butterfield CE, Chaponis DM, Laforme AM, Folkman J, Kieran MW. Inhibition of tumor angiogenesis by oral etoposide. Exp Ther Med 2010;1:739-46.  [PUBMED] |
| Figures |


Lieber Heli!
Das Herunterladen dieses wichtigen Artikels funktioniert leider nicht. Kannst Du helfen?
L.G. Wolfgang
indisches Journal, die verschieben die Artikel ständig, desswegen hab ich denn Text im Einschub weiter unten zur Gänze reinkopiert